ph3 molecular geometry|ph3 boiling point : Baguio Although Phosphine or PH3 molecule resemble NH3molecule, there is a difference in their bond angles. The central atom . Tingnan ang higit pa Baguio Transient Rooms in Session Road, Baguio: See traveler reviews, candid photos, and great deals for Baguio Transient Rooms in Session Road at Tripadvisor.
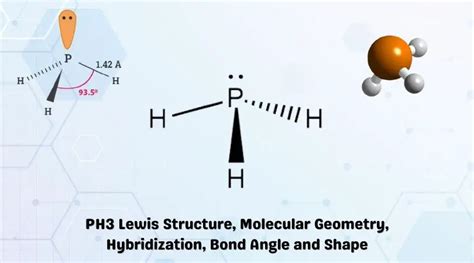
ph3 molecular geometry,In this molecule, Phosphorus has one lone pair of electrons along with three bonding pairs of electrons. According to the VSEPR theory, there are repulsive forces between bonding pairs of electrons, bonding and nonbonding pairs of electrons, and even nonbonding electrons. As a result, . Tingnan ang higit pa
Valence electrons are the electrons present in the outermost shell of the atom. These electrons are the ones that participate . Tingnan ang higit pa

Lewis Structure is the pictorial representation of the arrangement of atoms and valence electrons in the molecule. To know the Lewis Structure, we first know the central atom and the arrangement . Tingnan ang higit paAlthough Phosphine or PH3 molecule resemble NH3molecule, there is a difference in their bond angles. The central atom . Tingnan ang higit paThis may come as a surprise to some, but there is no defined hybridization for the Phosphine molecule. If you are aware of the Drago Rule, then you must know that we don’t . Tingnan ang higit pa
Learn how to draw the Lewis structure, molecular geometry, and hybridization of Phosphorous Trihydride (PH3), a compound of phosphorous and hydrogen atoms. Find out the electronic configuration, . A quick explanation of the molecular geometry of PH3 (Phosphorus trihydride) including a description of the PH3 bond angles. Note, the actual P-H bond angle is 93.5 degrees, rather.
PH3 is one of the easy molecules to understand the molecular geometry concept. Phosphorus Hydride or PH3 comprises one Phosphorus atom and three hydrogen atoms. For .Shape of PH 3 is trigonal pyramidal. Molecular geometry around phosphorous atom is tetrahedral. Total valence electrons pairs around phosphorous atom is four. In this .Learn how to draw and predict the PH3 molecular geometry using the VSEPR theory and molecular hybridization. Find out the number of lone pairs, valence electrons, bond . How to draw lewis structure for PH3? To write the PH3 lewis structure one should know the total of all the valence electrons that could be present in the molecule .
What is the molecular geometry of PH3? PH3 has a trigonal pyramidal molecular geometry, meaning it adopts a shape resembling a pyramid with three surrounding .ph3 molecular geometry ph3 boiling pointPhosphine (PH 3) Molecule Shape, Geometry, Hybridization. Lewis structure of Phosphine molecule contains three sigma bonds and one lone pair around phosphorus atom. .
Chemistry Learning Made Easy: PH3 Lewis Structure and Molecular Geometry. chem101csub. 3.83K subscribers. Subscribed. 13. 6K views 10 years ago. . Hello Guys!PH3 is one of the easy molecules to understand the molecular geometry concept. Phosphorus Hydride or PH3 comprises one Phosphorus atom and three . Geometry of Molecules. Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity.We recommend using the latest version of Chrome, Firefox, Safari, or Edge. Explore molecule shapes by building molecules in 3D! How does molecule shape change with different numbers of bonds and electron pairs? Find out by adding single, double or triple bonds and lone pairs to the central atom. Then, compare the model to real molecules!
1 Lone Pair. These are of the form AX 3 E and have trigonal pyramidal molecular geometries. Note the bond angle is less than the ideal because the lone pair take up more space. Figure 8.6.5 8.6. 5: Molecules like ammonia have tetrahedral electronic geometry but trigonal pyramidal molecular geometry. We can use the VSEPR model to predict the geometry of most polyatomic molecules and ions by focusing on only the number of electron pairs around the central atom, ignoring all other valence electrons present.According to this model, valence electrons in the Lewis structure form groups, which may consist of a single bond, a . H2S Molecular geometry. Hybridization of the given molecule H2S is sp3; the Sulfur atom is in center bonding with two Hydrogen atoms forming the bond angle less than 180 degrees. According to the VSEPR theory, the lone pairs of electrons repel each other, but as the Sulfur atom is less electronegative, the bond angle decreases to 104.5 .
A bond distance (or bond length) is the distance between the nuclei of two bonded atoms along the straight line joining the nuclei. Bond distances are measured in Ångstroms (1 Å = 10 –10 m) or picometers (1 pm = 10 –12 m, 100 pm = 1 Å). Figure \boldsymbol10.3.1 \boldsymbol 10.3. 1: Bond distances (lengths) and angles are shown for the .Species in the CCCBDB. Mostly atoms with atomic number less than than 36 (Krypton), except for most of the transition metals. See section I.B.1 for a periodic table view. Six or fewer heavy atoms and twenty or fewer total atoms. Exception: Versions 8 and higher have a few substituted benzenes with more than six heavy atoms.In essence, ph 3 is a Drago molecule and if we look at its bond angle data it shows that the p-orbitals have an angle of 90°. Looking at its Lewis structure we can state that molecular geometry of PH 3 is trigonal pyramidal. Important Points To Remember. In PH 3 hybridization does not take place. The pure p orbitals take part in bonding. PF3 is a tetra-atomic molecule where phosphorus donates three valence electrons, and three fluorine atoms accept one electron each to undergo a bond formation and reach a stable condition. Below are the steps to draw the lewis structure of the PF3 molecule. 1. Find out the total number of valence electrons in PF3, which is 26.Lewis electron structures give no information about molecular geometry, the arrangement of bonded atoms in a molecule or polyatomic ion, which is crucial to understanding the chemistry of a molecule. The valence-shell electron-pair repulsion (VSEPR) model allows us to predict which of the possible structures is actually observed in most cases.Do you want to build a molecule in 3D? Try this interactive simulation and learn how to create different molecular shapes by adding or removing atoms and bonds. Compare your results with real molecules and discover the principles of molecular geometry.
Steps to form OF2 Lewis Structure Diagram. Step 1: Find the Total number of Valence Electrons. The first and foremost step is to calculate the total number of valence electrons in an OF2 molecule. .ph3 molecular geometry In this article, we will discuss Phosphorous trifluoride (PF3) lewis structure, molecular geometry or shape, electron geometry, hybridization, polar or nonpolar, its bond angle, etc. “Phosphorus trifluoride is similar to carbon monoxide in that it is a gas which strongly binds to iron in hemoglobin, preventing the blood from absorbing oxygen.”.ph3 boiling pointPhosphine (PH 3) Molecule Shape, Geometry, Hybridization. Lewis structure of Phosphine molecule contains three sigma bonds and one lone pair around phosphorus atom. Therefore, there are total of four electrons regions. So, hybridization of phosphorus atom is sp 3.Because there are four electrons regions, geometry is tetrahedral and shape is .
Geometry of the water molecule with values for O-H bond length and for H-O-H bond angle between two bonds. Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule.It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters . The CH3Cl is a Penta atomic molecule with a bond angle of 109.5° which gives the molecule a bent shape. The molecular geometry of a molecule can be studied with the help of the Valence Shell Electron Pair Repulsion (VSEPR) theory which says chloromethane (CH3Cl) has a tetrahedral shape as the bond angle is 109.5° with the .We can use the VSEPR model to predict the geometry of most polyatomic molecules and ions by focusing on only the number of electron pairs around the central atom, ignoring all other valence electrons present.According to this model, valence electrons in the Lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, .
ph3 molecular geometry|ph3 boiling point
PH0 · ph3 boiling point
PH1 · molecular geometry practice quiz
PH2 · molecular geometry list
PH3 · molecular geometry chart
PH4 · how to determine molecular geometry
PH5 · how to determine electron geometry
PH6 · experimental bond lengths of ph3
PH7 · ch3 electron pair geometry
PH8 · Iba pa